
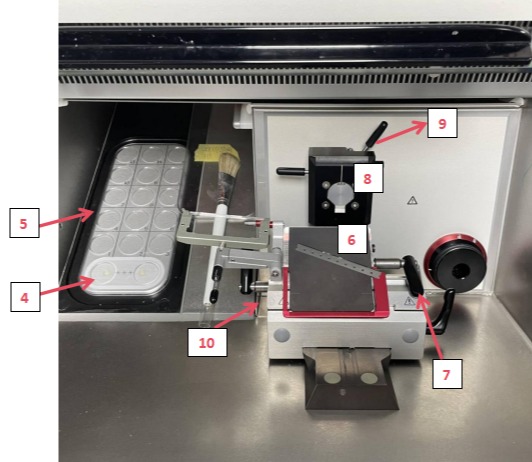
I. Parameters
1.Control Panel 1: Controls specimen retrieval, temperature, time, lighting, and UVC sterilization.
2. Control Panel 2: Manages motorized coarse feed for adjusting sectioning and trimming thickness.
3. Handwheel: Rotate for sectioning; lock in the 12 o’clock position after use.
4. Peltier Element (dual-station): Rapidly and precisely cools specimens. Both stations independently chill samples to optimal low temperatures for high-quality, intact sections.
5. Freezing Stage: Holds multiple specimen holders for organized storage, ensuring samples remain in a stable environment.
6. CE Knife Holder: Secures the microtome blade. Stability is critical for sectioning quality.
7. Knife Holder Adjustment: Quickly adjusts the distance between the specimen head and blade for preliminary positioning, improving efficiency.
8. Specimen Positioning Device: Aligns the specimen’s position and angle to target specific regions for sectioning.
9. Specimen Head Adjustment: Fine-tunes the specimen’s angle to achieve optimal orientation for precise sectioning of target areas.
10. Waste Collection Tray: Collects debris during sectioning to maintain workspace cleanliness and prevent interference.
II. Operating Instructions
1. Power On: Start the instrument at least 5 hours before use. Press and hold the key button and "+" on the control panel to activate the specimen head area. Turn on LED lighting.
2. Preparation: Maintain the chamber and specimen head at -15°C to -20°C. Lower temperatures may cause tissue brittleness. Adjust section thickness based on tissue size and hardness.
3. Mount Specimen Holder**: Apply OCT embedding compound to the holder, place the embedded tissue, add another OCT layer after solidification, and freeze on the freezing stage. Secure the holder on the specimen clamp.
4. Blade Installation: Mount and secure the blade.
5. Trimming: Adjust the specimen-blade distance. Perform rough cutting to smooth the tissue surface. Remove debris with a brush before reducing thickness for sectioning.
6. Sectioning: Lower the anti-roll plate. Cut sections at 30–50 μm with moderate speed. Monitor the cutting plane and adjust if tilting occurs.
7. Section Storage: Place sections in a cell culture plate and rinse with PBS buffer for downstream procedures.
8. Cleaning: Remove the blade and clear the waste tray.
9. Power Off: Press and hold the key button and "-" on the control panel to deactivate the specimen head. Turn off LED lighting.
10. Lock Handwheel: Rotate the handwheel to the 12 o’clock position and lock it.